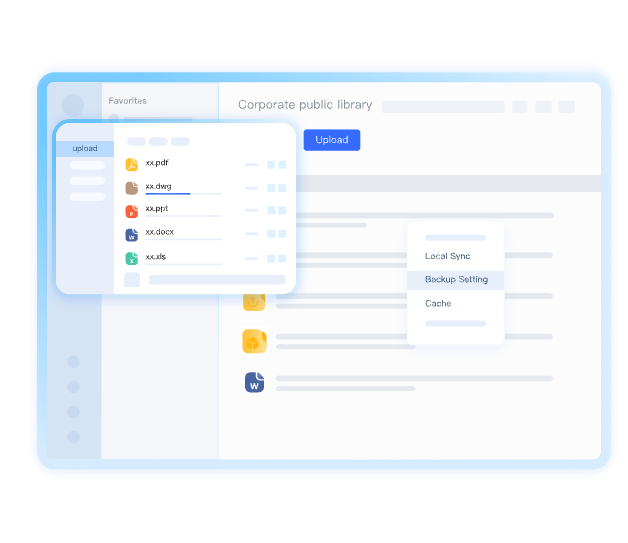
Centralize All Research Documents
Securely centralize drug development files, clinical trial data, and patent materials in a single compliant platform with version control and automated backups.
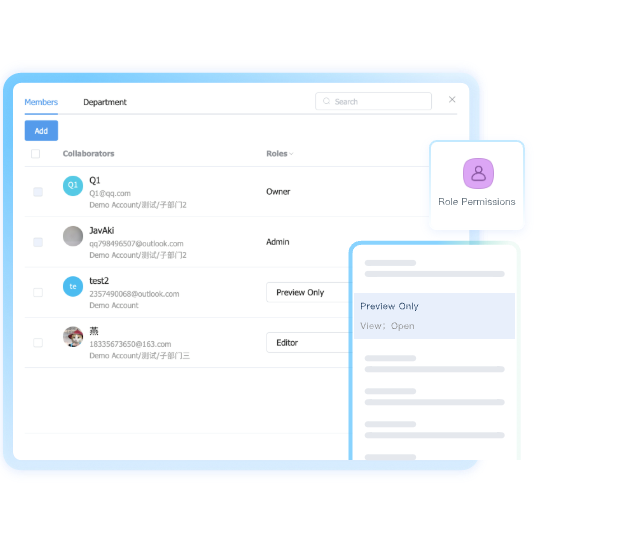
Protect Sensitive Research Data
Implement role-based access controls and activity monitoring to safeguard formulas, trial results, and product specifications throughout their lifecycle.
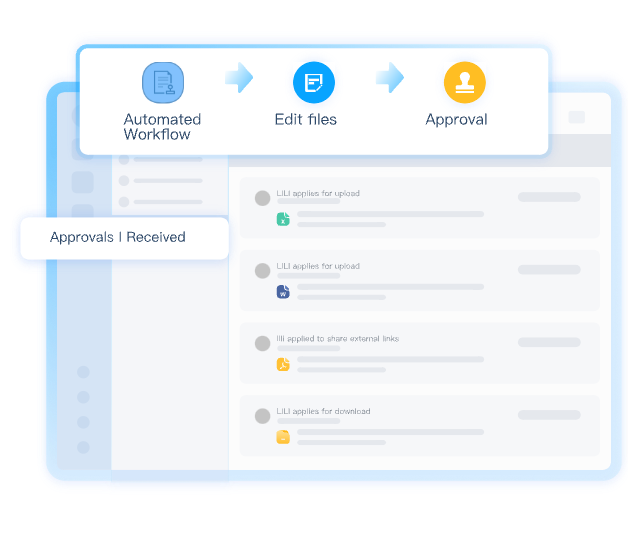
Manage Compliant Approvals
Track and control document reviews, modifications, and approvals with full audit capabilities to meet pharmaceutical regulations.